-
Healthcare Professionals
-
Therapies and Procedures
-
Concomitant Surgical Ablation Therapy
- Ablation Sensing Unit & Switch Matrix
- cryoFORM® Cryoablation Probe
- cryoICE® BOX V6
- cryoICE® Cryoablation Probes
- Isolator® Linear Pen
- Isolator® Synergy™ Access® Clamp
- Isolator® Synergy™ Clamps (OLL2/OSL2)
- Isolator® Transpolar Pen (MAX3)
- Isolator® Synergy™ EnCompass® Clamp
- Multifunctional Ablation Generator (MAG)
- Hybrid AF™ Therapy
- Hybrid Total Thoracoscopic Therapy
- Left Atrial Appendage Management
- Cryo Nerve Block Therapy
-
Concomitant Surgical Ablation Therapy
- Education & Training
- Clinical Evidence
- Product Labeling
- Resources
- Society Guidelines
-
Therapies and Procedures
- Patients & Caregivers
- About AtriCure
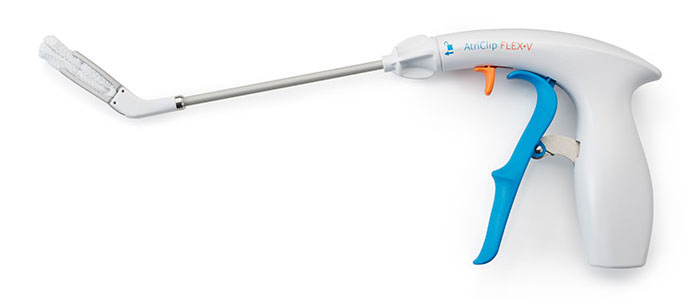
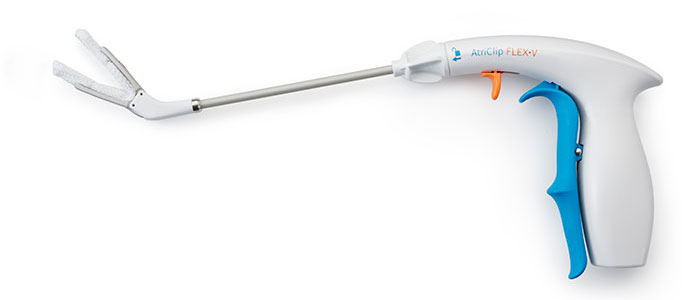
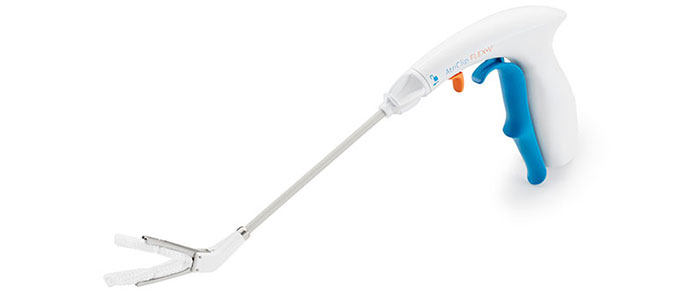
AtriClip FLEX•V Device
A Simplified Approach to Left Atrial Appendage Exclusion
Request More InformationThis product may not be available in your specific country. Contact your local AtriCure representative to check availability.
Product Features
- Tip first closure with continuous dynamic closing pressure
- Intentionally designed with Dacron to support atraumatic placement and security in location
- One-handed application with reduced fatigue clip opening lever
- Suture-less clip deployment

The AtriClip LAA Exclusion System is indicated for use in patients at high risk of thromboembolism for whom left atrial appendage exclusion is warranted.
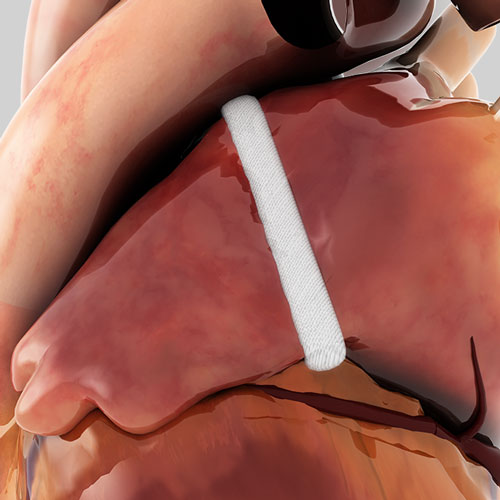
Isolation of Appendage
The AtriClip family of devices are the only Left Atrial Appendage Management (LAAM) devices clinically proven to provide both mechanical1 and electrical isolation of the LAA.2
Class I Recommendation by ESC/EACTS
In the 2024 ESC/EACTS AF Guidelines, surgical LAA Exclusion has been upgraded to a Class I Recommendation, joining the highest recommendation level shared from ACC/AHA/ACCP/HRS Society Guidelines.
Reduce the Risk of Stroke* with LAAE
By excluding the LAA, the risk of future *thromboembolic events originating from the LAA is reduced for the patient. AtriCure’s AtriClip devices are the most widely implanted LAAM devices in the world, providing a permanent solution to LAA exclusion during cardiac procedures.
650,000+
AtriClip Devices Sold
20+
Years of Expertise and Innovation in LAAM
90+
Published Studies Including AtriClip Devices
PRODUCT CODES:
- ACHV35 (35 mm)
- ACHV40 (40 mm)
- ACHV45 (45 mm)
- ACHV50 (50 mm)
Page References
- Data on file.
- Starck, C.T. et al. (2012). Epicardial left atrial appendage clip occlusion also provides the electrical isolation of the left atrial appendage. Interactive cardiovascular and thoracic surgery, 15(3):416-18. This human clinical study demonstrated acute electrical isolation. Chronic electrical isolation has not been evaluated in human clinical studies.
MRI SAFETY INFORMATION:
MR CONDITIONAL
Non-clinical testing demonstrated that the LAA Exclusion System Clip is MR Conditional. A patient with this device can be scanned safely in an MR system immediately after placement under the following conditions:
- Static magnetic field of 1.5-Tesla and 3-Tesla.
- Maximum spatial gradient magnetic field of 4,000-gauss/cm (40-T/m).
- Maximum MR system reported, whole body averaged specific absorption rate (SAR) of 4-W/kg for 15 minutes of scanning (i.e., per pulse sequence) in the First Level Controlled Operating Mode of operation for the MR system.
- The scan conditions defined for the LAA Exclusion System Clip are expected to produce a maximum temperature rise of 2.9°C (5.22°F) for Gillinov-Cosgrove Clip (LAA, ACH1, ACH2, PRO1, PRO2 devices) and 3.1°C (5.58°F) for V Clip (FLEX·V, PRO·V devices) after 15 minutes of continuous scanning (i.e., per pulse sequence).
PM-EUMEA-2693B-1026-G