-
Healthcare Professionals
-
Therapies and Procedures
-
Concomitant Surgical Ablation Therapy
- Ablation Sensing Unit & Switch Matrix
- cryoFORM® Cryoablation Probe
- cryoICE® BOX V6
- cryoICE® Cryoablation Probes
- Isolator® Linear Pen
- Isolator® Synergy™ Access® Clamp
- Isolator® Synergy™ Clamps (OLL2/OSL2)
- Isolator® Transpolar Pen (MAX3)
- Isolator® Synergy™ EnCompass® Clamp
- Multifunctional Ablation Generator (MAG)
- Hybrid AF™ Therapy
- Hybrid Total Thoracoscopic Therapy
- Left Atrial Appendage Management
- Cryo Nerve Block Therapy
-
Concomitant Surgical Ablation Therapy
- Education & Training
- Clinical Evidence
- Product Labeling
- Resources
- Society Guidelines
-
Therapies and Procedures
- Patients & Caregivers
- About AtriCure
Left Atrial Appendage Management
AtriCure’s AtriClip Products are the Most Widely Sold Left Atrial Appendage (LAA) Management Devices Worldwide
The AtriClip devices are epicardially applied to the base of the left atrial appendage (LAA) during cardiac surgery procedures such that the LAA is permanently excluded from the left atrium of the heart. This exclusion eliminates blood flow and electrical communication of blood between the left atrium (LA) and the LAA and results in the electrical isolation of the LAA from the rest of the heart.1-4
LAA Exclusion Upgraded to Class I Recommendation by ESC/EACTS
In the 2024 ESC/EACTS AF Guidelines7, surgical LAA Exclusion has been upgraded to a Class I Recommendation, joining the highest recommendation level shared from ACC/AHA/ACCP/HRS Society Guidelines.
Learn More About Society Guidelines
LAA Exclusion has been shown to reduce the relative LAA-related stroke by over 40%13.
Thrombus in the Left Atrial Appendage (LAA) is believed to be the primary cause of stroke in AF patients8-12. In a review of 23 studies8, 91% of thrombus formations were located in the LAA.
LAAE has been shown to reduce ischemic stroke risk for AF patients undergoing concomitant surgery. The results of the LAAOS III randomized control trial13 show that concomitant LAAE improves outcomes compared with no-LAAE in AF patients. Results showed a lower risk of ischemic stroke or systemic embolism in AF patients with LAAE performed during surgery than without it.
Hallmarks of AtriClip Device Excellence
Epicardial Exclusion
- Implant is not in the blood stream
- Ischemic injury electrically isolates the LAA
- Atrophy and absorption of the LAA
Continuous Dynamic Closing Pressure
- Continuous closing force maintains LAA exclusion throughout changes to the tissue caused by ischemia.
Parallel / Linear Closing
- Minimized occurrence of tissue folds with optimal apposition of tissue along long axis of LAA ostium
Tissue Compression / Atraumatic
- No cutting, non-piercing, and atraumatic compression reduces risk of tissue tearing and bleeding
The AtriClip devices are available on a variety of delivery systems optimized for open concomitant procedures as well as minimally invasive surgery, both through a thoracotomy and a port.
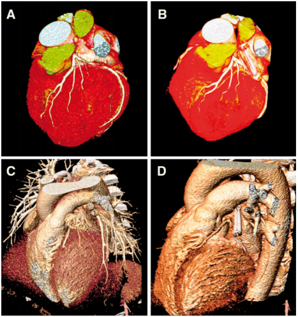
Computed tomography (CT) before and at 3-year follow-up after AtriClip device implantation. Exemplary CT before AtriClip device placement (A and C) and after implantation depicting the AtriClip device in stable position and fully excluding the left atrial appendage at a 3-year follow up (B and D).5
*Chronic electrical isolation has not been evaluated in human clinical studies.
PM-EU-3740A-0926-G
PM-EU-3739A-0926-G
Learn More About the
AtriClip LAA Exclusion System
Page References
References:
- Benussi, S. et al, Thoracoscopic Appendage Exclusion With an AtriClip Device As a Solo Treatment for Focal Atrial Tachycardia. Circulation, 2011, 123:1575-8.
- Starck, CT et al, Epicardial left atrial appendage clip occlusion also provides the electrical isolation of the left atrial appendage. Interactive Cardiovascular and Thoracic Surgery, 2012, 15(3):416-419.
- Kamohara K et al. A novel device for left atrial appendage exclusion. J Thorac Cardiovasc Surg 2005, 130(6):1639-44.
- Kamohara K et al, Evaluation of a novel device for left atrial appendage exclusion: The second-generation atrial exclusion device, J Thorac Cardiovasc Surg, 2006, 132:340-6.
- Toale C, Fitzmaurice GJ, Eaton D, Lyne J, Redmond KC. Outcomes of left atrial appendage occlusion using the AtriClip device: a systematic review. Interact CardioVasc Thorac Surg 2019; doi:10.1093/icvts/ivz156.
- Fumoto H. et al. (2008). A novel device for left atrial appendage exclusion: the third-generation atrial exclusion device. J Thorac Cardiovasc Surg, 136(4):1019-27.
- 2024 ESC Guidelines for the management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): Developed by the task force for the management of atrial fibrillation of the European Society of Cardiology (ESC), with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Endorsed by the European Stroke Organisation (ESO). European Heart Journal, ehae176, doi.org/10.1093/eurheartj/ehae176.
- Blackshear JL, Odell JA. Appendage obliteration to reduce stroke in cardiac surgical patients with atrial fibrillation. Ann Thorac Surg. 1996;61(2):755-759.
- Glikson M, Wolff R, Hindricks G, et al. EHRA/EAPCI expert consensus statement on catheter-based left atrial appendage occlusion – an update. EuroIntervention. 2020;15(13):1133-1180.
- Hanke T. Surgical management of the left atrial appendage: a must or a myth? Eur J Cardiothorac Surg. 2018;53(suppl_1):i33-i38.
- Johnson WD, Ganjoo AK, Stone CD, Srivyas RC, Howard M. The left atrial appendage: our most lethal human attachment! Surgical implications. Eur J Cardiothorac Surg. 2000;17(6):718-722.
- van Laar C, Verberkmoes NJ, Es HW, et al. Thoracoscopic left atrial appendage clipping. JACC: Clinical Electrophysiology. 2018;4(7):893-901.
- Whitlock RP, Belley-Cote EP, Paparella D, et al. Left Atrial Appendage Occlusion during Cardiac Surgery to Prevent Stroke. N Engl J Med. 2021;384(22): 2081-2091.
- Emmert et al, Safe Effective and Durable Epicardial Left Atrial Appendage Clip Occlusion in Patients with AF Undergoing Cardiac Surgery: First Long-term Results from a Prospective Device Trial. European Journal of Cardio-Thoracic Surgery, 2014 Jan;45(1): 126-31.
Warning:
The safety and effectiveness of this device in atrial rhythm control management, either alone or in combination with ablative treatment, has not been established.
The safety and effectiveness of this device for stroke prevention, either alone or in combination with cardiac surgery, has not been established.
*AtriClip PRO•V Device is not included.
PM-EUMEA-3691A-1026-G

