-
Healthcare Professionals
-
Therapies and Procedures
-
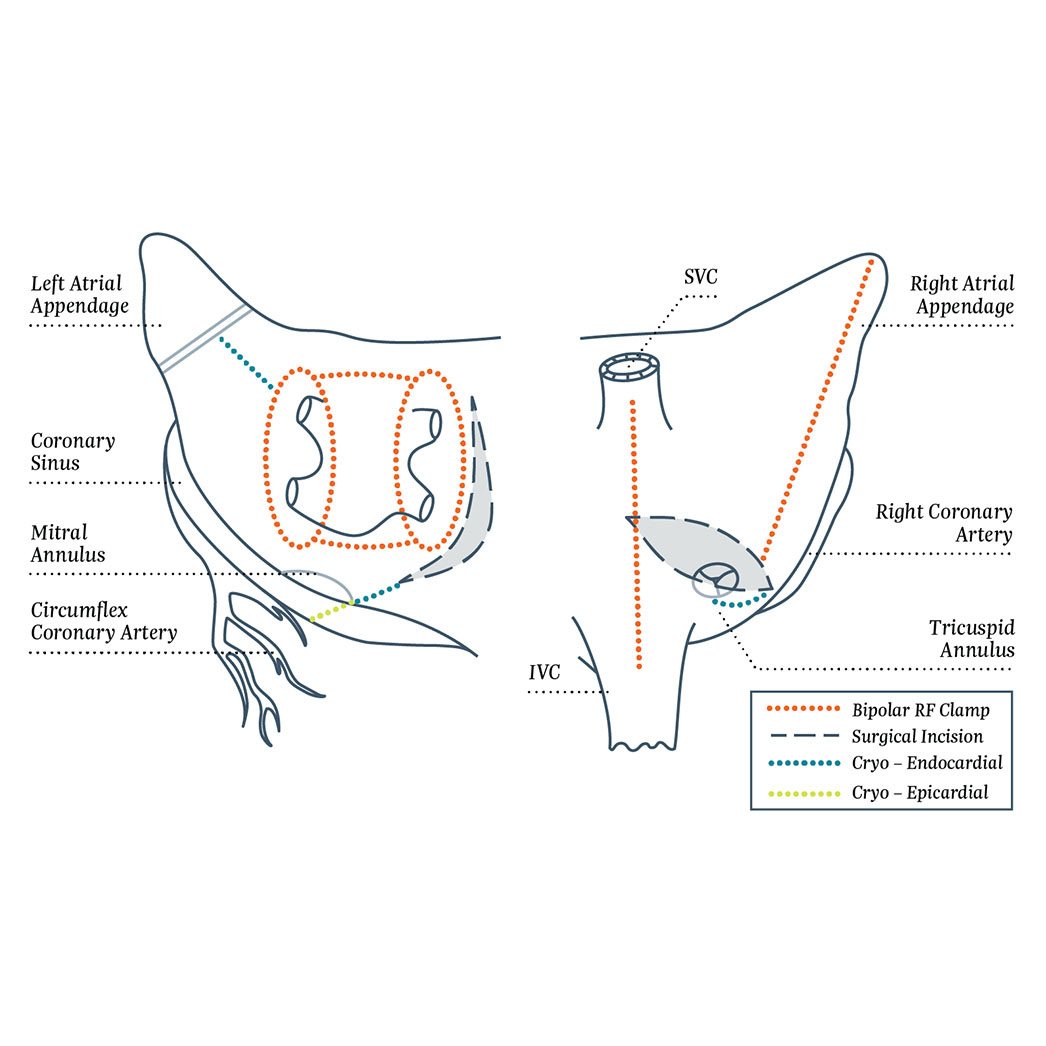
Concomitant Surgical Ablation Therapy
- Ablation Sensing Unit & Switch Matrix
- cryoFORM® Cryoablation Probe
- cryoICE® BOX V6
- cryoICE® Cryoablation Probes
- Isolator® Linear Pen
- Isolator® Synergy™ Access® Clamp
- Isolator® Synergy™ Clamps (OLL2/OSL2)
- Isolator® Transpolar Pen (MAX3)
- Isolator® Synergy™ EnCompass® Clamp
- Multifunctional Ablation Generator (MAG)
- Hybrid AF™ Therapy
- Hybrid Total Thoracoscopic Therapy
- Left Atrial Appendage Management
- Cryo Nerve Block Therapy
-
Concomitant Surgical Ablation Therapy
- Education & Training
- Clinical Evidence
- Product Labeling
- Resources
- Society Guidelines
-
Therapies and Procedures
- Patients & Caregivers
- About AtriCure
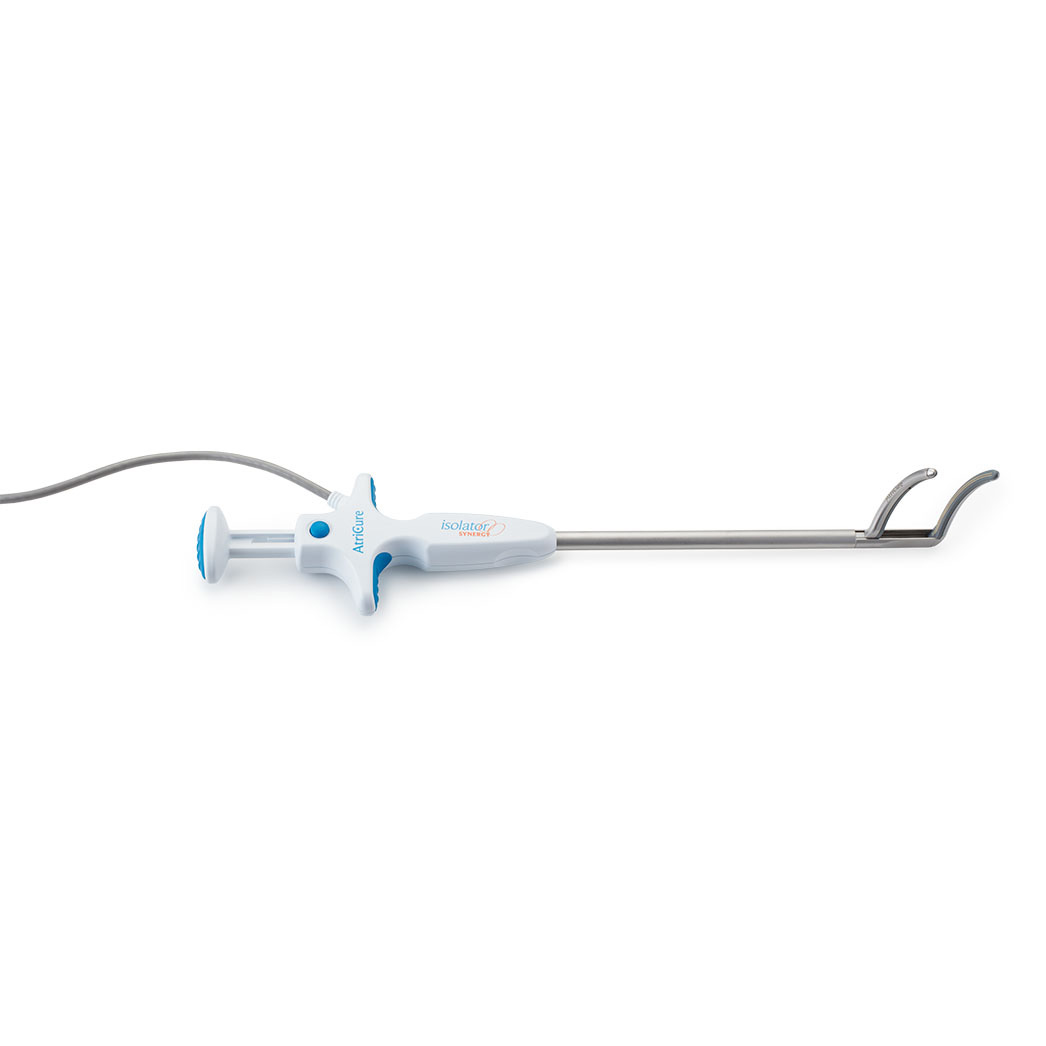
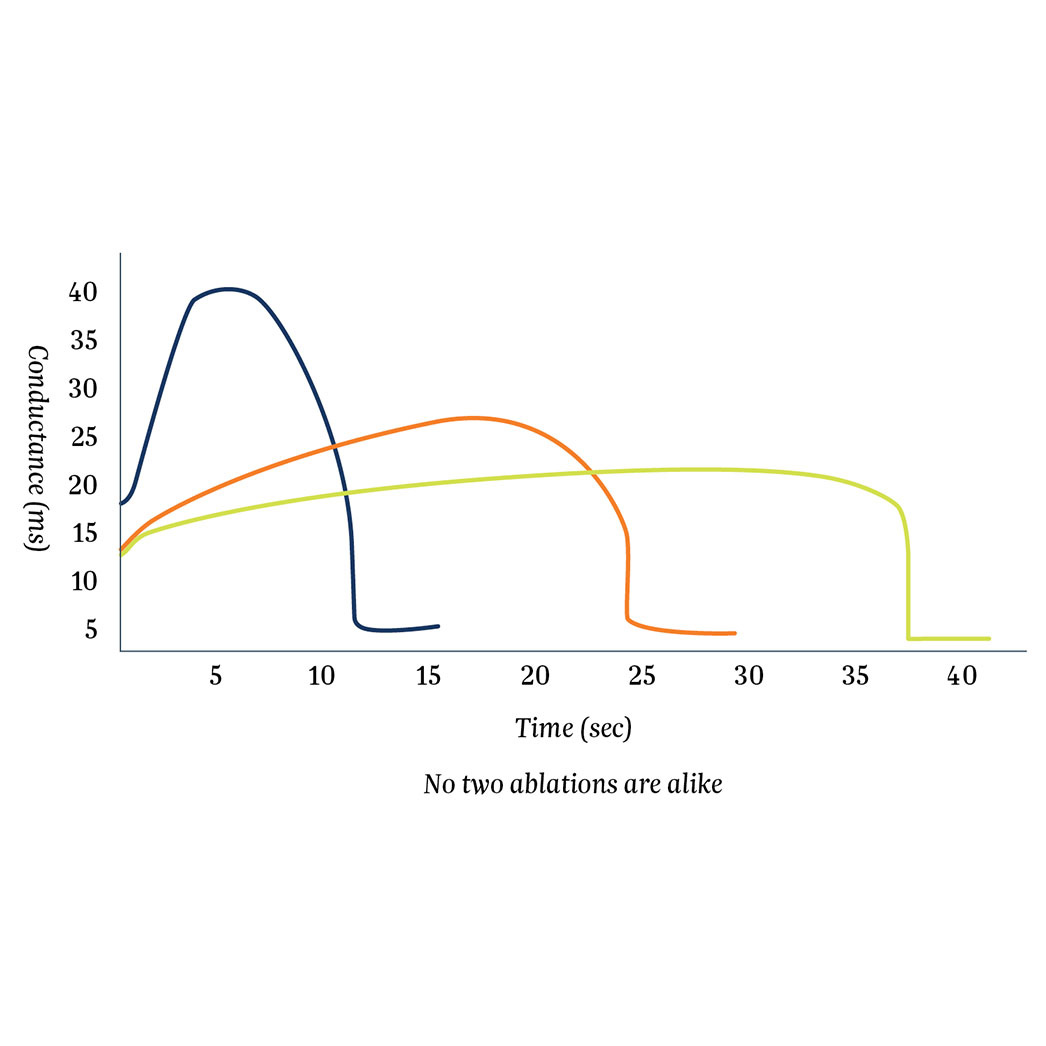
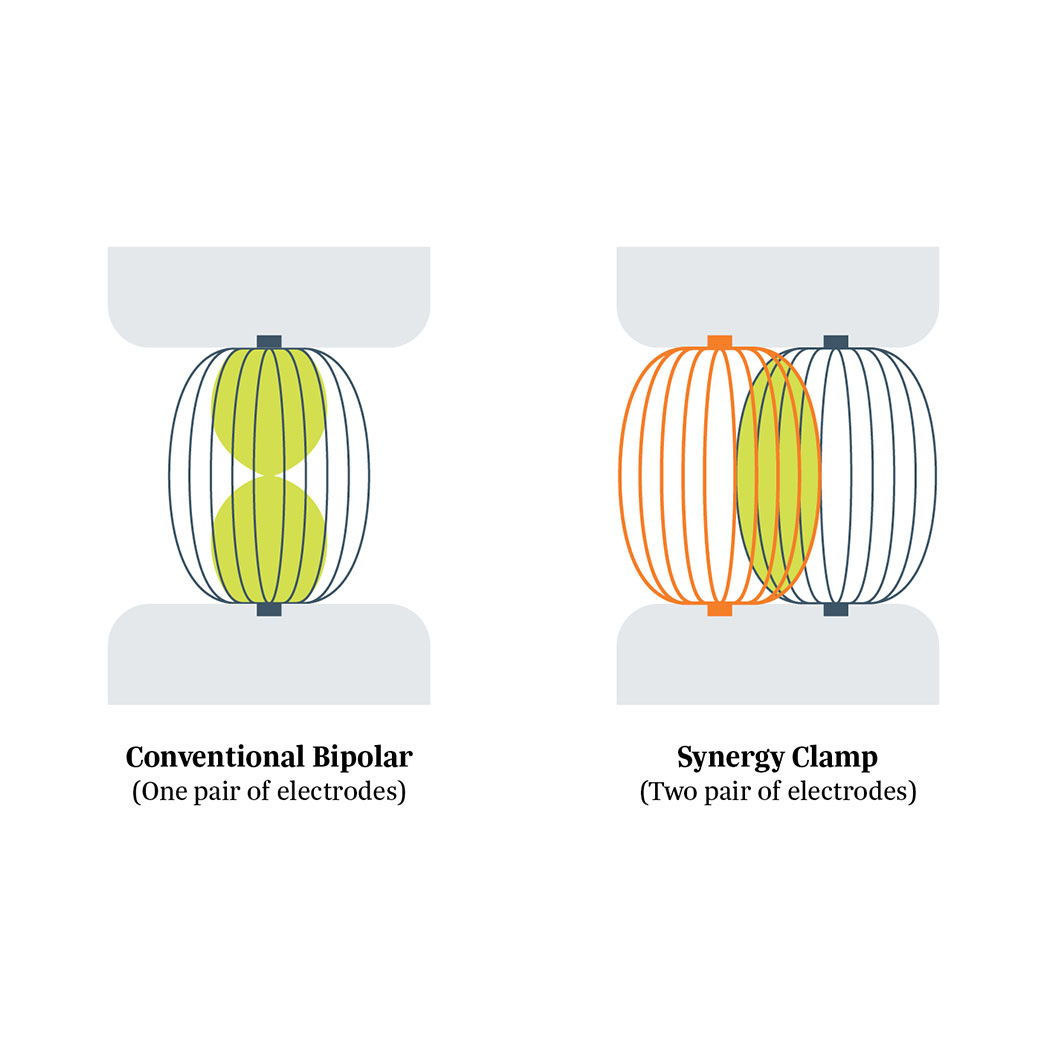
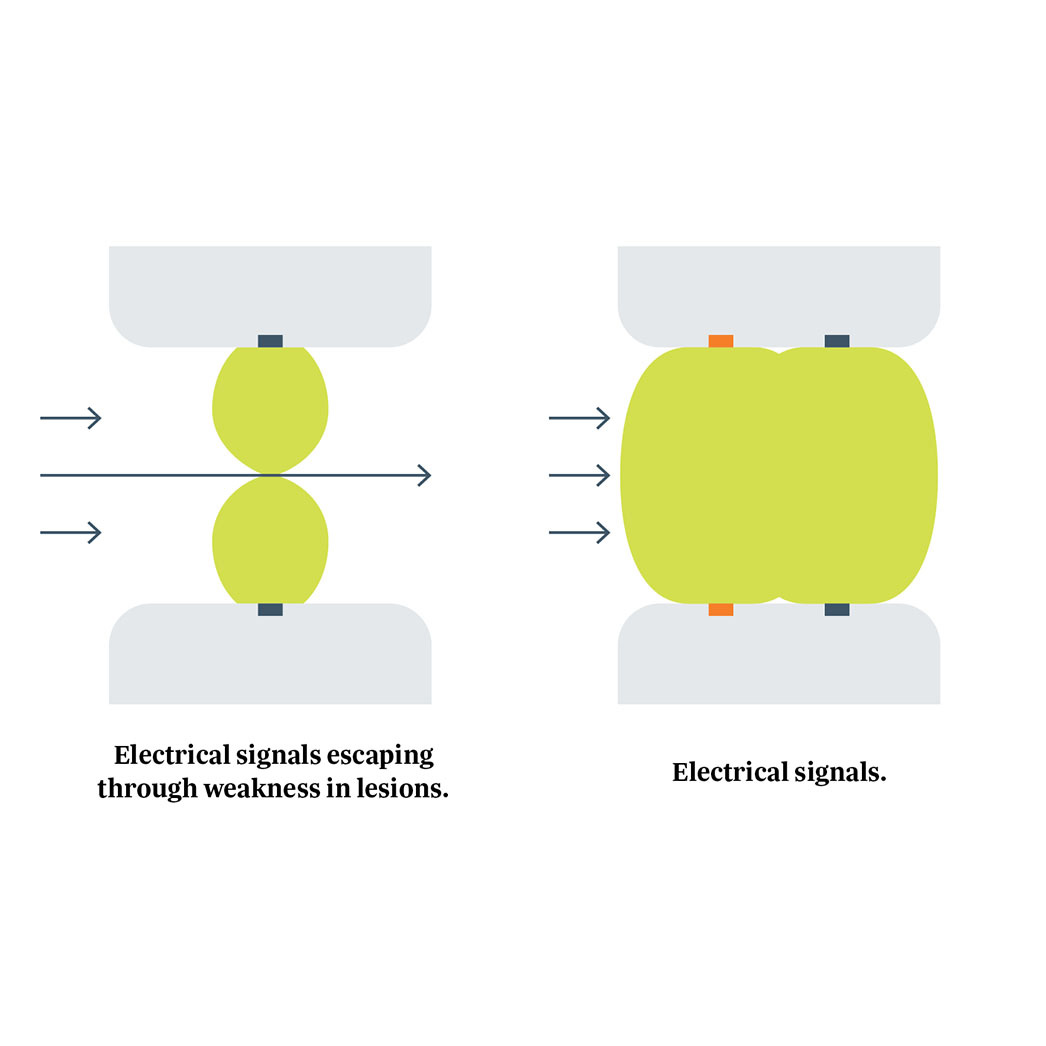
Isolator Synergy Clamps (OLL2/OSL2)
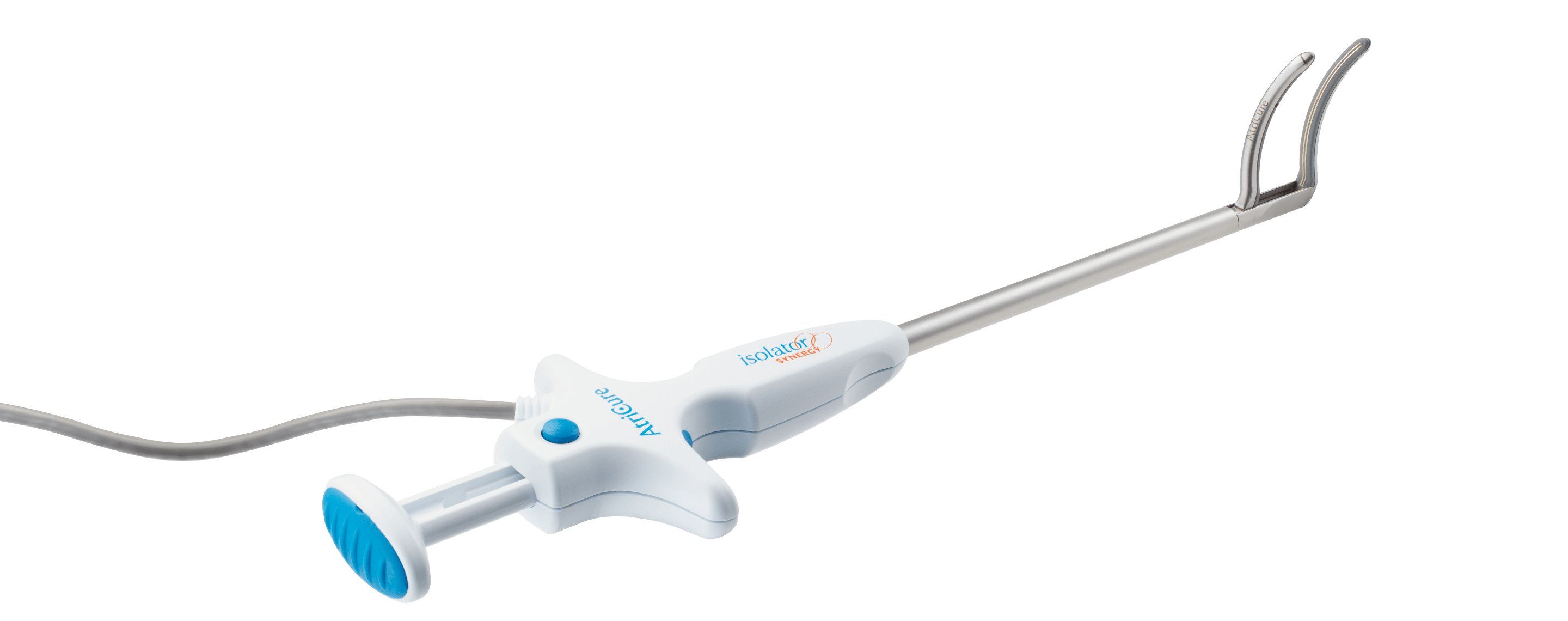
This product may not be available in your specific country. Contact your local AtriCure representative to check availability.

Available Downloads
PM-INTL-2676B-0927-G